Functional Group
Nomenclature
The suffix for amides in "-anamide".
Like other carboxylic acid derivatives, the amide is on the terminal carbon. Any other groups bound to the nitrogen atom (secondary and tertiary amides) are named as, for example, N-methyl.
N,N-dimethyl methanamide
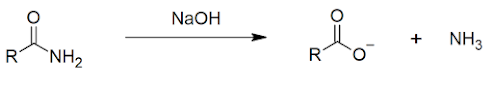
Preparation
FROM CARBOXYLIC ACIDS
Heating is a critical step in this, to drive off the water and form the amide bond. This is called a condensation reaction.





No comments:
Post a Comment