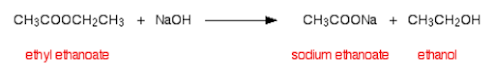
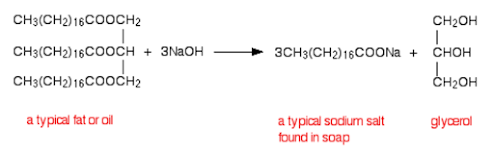
Carboxylic Acids are another important organic compound. This is primarily because they give compounds characteristic properties (being that they are weak acids), and they are also important precursors to other useful organic functional groups (amides and esters, especially).
Nomenclature
The carboxylic acid functional group is always on the terminal carbon (the carbon that we number as "1"). We name the compound from that carbon chain length, and any additional functional groups and/or side chains:
Preparation
Carboxylic acids are prepared (made) by oxidising a primary alcohol under reflux.
Reactions of Carboxylic Acids
Carboxylic acids have all of the same reactions as other acids:
- with metals to produce hydrogen gas and a metal salt (e.g. magnesium ethanoate)
- with oxides/hydroxides to produce water and a salt (neutralisation)
- with carbonates/hydrogen carbonates to produce water, carbons dioxide and a salt
Their reaction rates are low because they are weak acids (lower hydronium ion concentrations than strong acids).
Their organic reactions are:
1. CHLORINATION
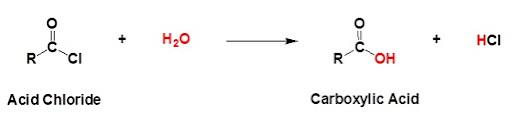
The reaction with thionyl chloride will produce an acid chloride:
Like chlorination of an alcohol, this is an example of nucleophilic substitution. As two small molecules (sulfur dioxide and hydrogen chloride) are produced, it could also be considered to be a condensation reaction.
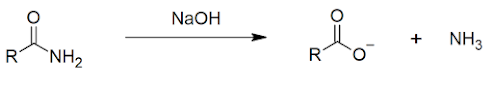
2. AMIDE PRODUCTION
Another form of condensation reaction produces and amide:
Note that there are two steps in this reaction. Without the additional heat, the reaction would stop at the production of the ammonium salt (ammonium propanoate).
3. ESTERIFICATION
There are two ways to make esters. Making them from carboxylic acids is the more difficult, but safer, way. This is another example of a condensation reaction, as a small molecule (water) is removed to make the compound:
Note: Concentrated sulfuric acid is used as it is a dehydrating agent. It removes the water, driving the equilibrium in the forward direction.