- aldehydes
- ketones
- carboxylic acids
- acid chlorides
- esters
- amides
The functional groups that contain this bond have key similarities and differences in their spectra. All of these notes assume that one of these functional groups is a possibility, either to be confirmed or to be excluded.
IR
One of the first things we should look for is the C=O stretch. This does not help us differentiate between the different carbonyl compounds, but it does confirm or exclude them (by its presence, or absence, respectively).
The C=O stretch will be strong (a large, obvious peak) and somewhere between 1600 and 1800 cm-1, depending upon the functional group. You can check it with your datasheet (provided in an assessment).
We then use other characteristic stretches and bends to narrow down which functional groups is most likely to be present. For example, two O=C-H bands around 2750 cm-1 for an aldehyde.
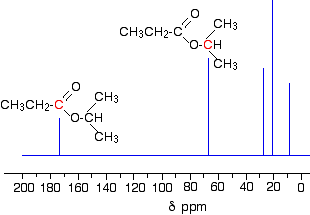
13C-NMR
All carbonyl compounds has a shift due to the C=O environment. Usually, the exact position of this shift allows us to narrow our options down, as not all of these functional groups have shift values that overlap. For example, a shift at 190 ppm is very unlikely to be a ketone (200-230 ppm), ester (155-185 ppm) or amide (150-170 ppm).
NOTE: These values have been estimated from the Spectroscopy Datasheet that we use in class and for our assessment tasks.
We then use the other shifts to infer which functional group is present. Again, there is some overlap in these, so you need to integrate your inferences from IR and NMR.
Mass Spec
There are not many characteristic fragments that we can use in Mass Spec. However, here are some that we should look for:
ALDEHYDES: M+ -29. This will usually be a large peak due to the removal of a O=C-H (CHO) fragment. It is not large in other carbonyl compounds, as it is only due to the removal of CH3CH2 in other compounds.
CARBOXYLIC ACIDS: M+ -45 and at 45m/z. These are due to fragmentation between the COOH and the rest of the molecule. We would not expect to see these two fragments in the other carbonyl compounds.
AMIDES: Amides contain nitrogen. All nitrogen compounds have an odd value for their M+ ion. This alone is not enough, though, as there is another nitrogen-containing compound that we study - amines.
If they are present, they provide further evidence of the functional group. If they are absent, they should be mentioned as evidence to exclude possible structures/functional groups.
No comments:
Post a Comment